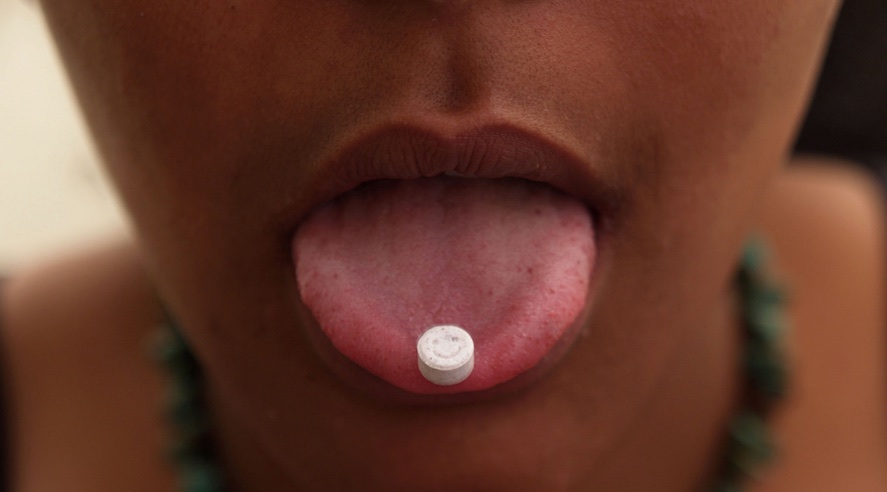
Do you constantly forget when — and if — you took your medication? A new type of pill just approved by the U.S. Food and Drug Administration might be right up your alley.
The FDA will allow the makers of Abilify MyCite aripiprazole tablets, a medication prescribed to treat schizophrenia and manic episodes, to sell the tablets outfitted with an ingestible sensor that “activates when it reaches stomach fluids and communicates with the patch,” according to the FDA. This allows the patients, their doctors and their families insight on when (and if) the medication was taken.
The medication is not tracked in real time. It takes anywhere from 30 minutes to two hours for the sensor to relay information back to a wearable patch, according to the FDA. The information is then accessible via a smartphone app, though there isn’t a 100 percent guarantee that the medicine will always track correctly.
The FDA says the pill’s tracking feature will be useful for certain types of patients, including adults with depression, bipolar disorders and schizophrenia, a devastating mental illness that affects up to one percent of adults in the United States.
“The FDA supports the development and use of new technology in prescription drugs and is committed to working with companies to understand how this technology might benefit patients and prescribers,” the FDA’s Mitchell Mathis said in a statement.
The trackable medication isn’t yet shown to improve patient compliance, according to the FDA, but taking it can give the patient and caregivers important information “with the goal of allowing physicians to be more informed in making treatment decisions that are specific to the patient’s needs.”
The sensor likely won’t change the size of the tablets, either: The sensor is about the size of a grain of sand.