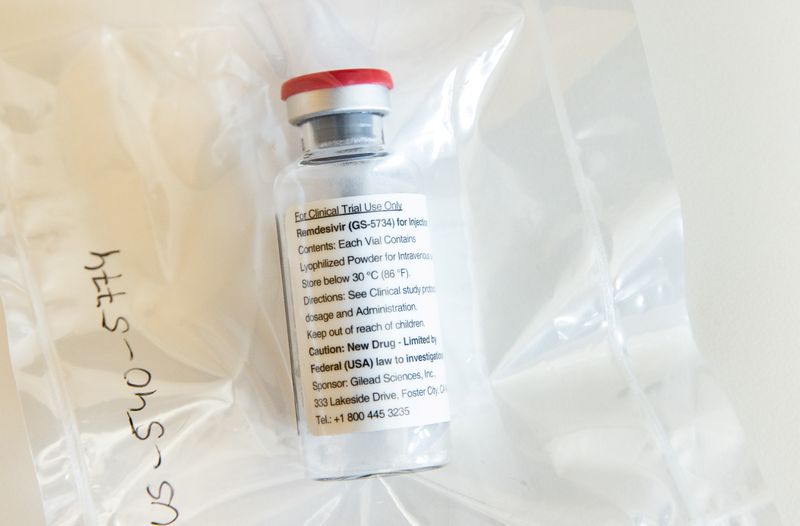
BERLIN (Reuters) – Germany has secured enough supplies for now of remdesivir, which is set to become the first COVID-19 treatment approved in Europe, and is banking on developer Gilead to meet future needs, the country’s health ministry said on Wednesday.
The U.S. Department of Health and Human Services (HHS) this week said it had secured all of Gilead’s projected production for July and 90% of its production in August and September, in addition to an allocation for clinical trials.
“The federal government has early on secured remdesivir for the treatment of coronavirus patients. Currently, there are still sufficient reserves,” Germany’s health ministry told Reuters in a written statement.
With a conditional market approval, which is expected to be issued by the EU Commission this week, comes an obligation to deliver sufficient quantities in the future, it added.
“We trust Gilead will meet this obligation,” the ministry said.
Once supplies are less constrained, HHS will stop managing the allocation, Gilead said at the time of the department’s statement.
Gilead has linked up with generic drugmakers based in India and Pakistan to supply remdesivir in 127 developing countries, but it has not discussed its supply strategy for developed nations outside the United States.
(Writing by Ludwig Burger; Editing by Jan Harvey)