BENGALURU (Reuters) – India has approved Merck’s COVID-19 pill and two more vaccines for emergency use as the world’s second most populous country braces for a possible spike in coronavirus cases due to the rapidly spreading Omicron variant.
Asia’s third-largest economy has already said it will allow COVID-19 booster shots for some of its population as some Indian states logged an uptick in Omicron cases.
The emergency approvals come at a time measures are being taken to ramp up oxygen supplies and strengthen the country’s health infrastructure.
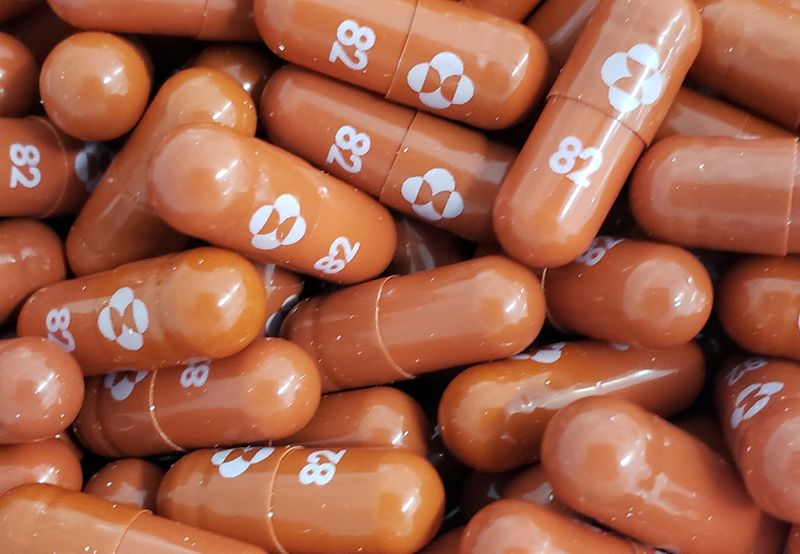
Molnupiravir will be manufactured in India by 13 companies for restricted use under emergency situation for treatment of adult patients with COVID-19, the country’s Health Minister Mansukh Mandaviya said on Tuesday.
Merck’s anti-viral pill molnupiravir was authorised by the United States last week for certain high-risk adult patients and has been shown to reduce hospitalisations and deaths by around 30% in a clinical trial.
Earlier this year, Aurobindo Pharma, Cipla, Sun Pharmaceuticals and some others signed non-exclusive voluntary licensing agreements with Merck to manufacture and supply molnupiravir in India.
Two other COVID-19 vaccines, Serum Institute of India’s version of Novavax Inc’s shot, Covovax, and homegrown drugmaker Biological E’s Corbevax were also granted emergency use approval, Mandaviya said on Twitter.
Medical experts have said India needs to double down on its vaccine campaign and some states have imposed night curfews and other restrictions in the run up to New Year festivities to prevent a spike in infections and a repeat of summer 2021 when a devastating second wave of infections left tens of thousands dead.
The country’s inoculation drive so far has been dominated by a domestically produced version of the AstraZeneca COVID-19 shot by Serum Institute and Bharat Biotech’s inactivated vaccine Covaxin.
India has so far administered 1.43 billion COVID-19 vaccine doses and 62% of its eligible population have received both doses. The country plans to start vaccinating those aged 15-18 from Jan. 3.
(Reporting by Chandini Monnappa in Bengaluru; Editing by Rupam Jain and Shounak Dasgupta)