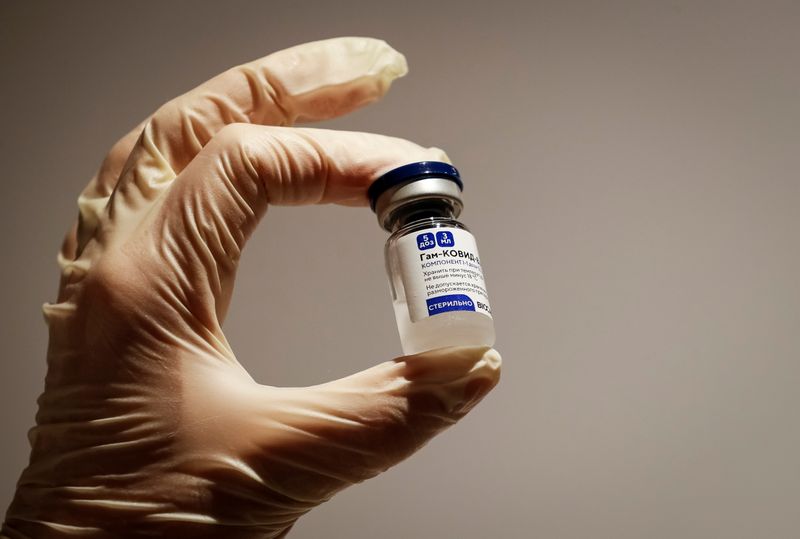
BENGALURU (Reuters) – Indian drugmaker Dr.Reddy’s Laboratories said on Friday it has begun the process of filing for emergency use authorization of Russia’s Sputnik V COVID-19 vaccine in India.
If approved, Sputnik V, developed by Moscow’s Gamaleya institute and marketed abroad by the Russian Direct Investment Fund (RDIF), will have the highest efficacy of vaccines currently cleared for emergency use in India.
AstraZeneca’s shot – which is manufactured by Serum Institute of India under the COVISHIELD brand name – and Bharat Biotech’s vaccine, COVAXIN, are the other two vaccines cleared for emergency use in the country.
The approval of the Sputnik V vaccine would bolster India’s immunisation campaign, which on Friday crossed the 10 million mark. The country aims to inoculate 300 million people by August.
Dr.Reddy’s has been working with RDIF to hold small domestic clinical studies of Sputnik V.
The vaccine has proved to be 91.6% effective against COVID-19 based on late-stage trials in Russia, a figure that has been confirmed by peer-reviewed results in The Lancet medical journal.
The drugmaker said it would present the safety profile of a mid-stage study and interim data from a late-stage trial, which is expected to be completed by Feb. 21, to the Drugs Controller General of India.
Shares of the company, which had fallen as much as 1.9%, reversed course to trade 1.4% higher following the news.
For comparison, Bharat Biotech is yet to release efficacy data for COVAXIN, while AstraZeneca’s vaccine has an efficacy of around 60%.
(Reporting by Chandini Monnappa and Anuron Kumar Mitra in Bengaluru; Editing by Aditya Soni and Anil D’Silva)