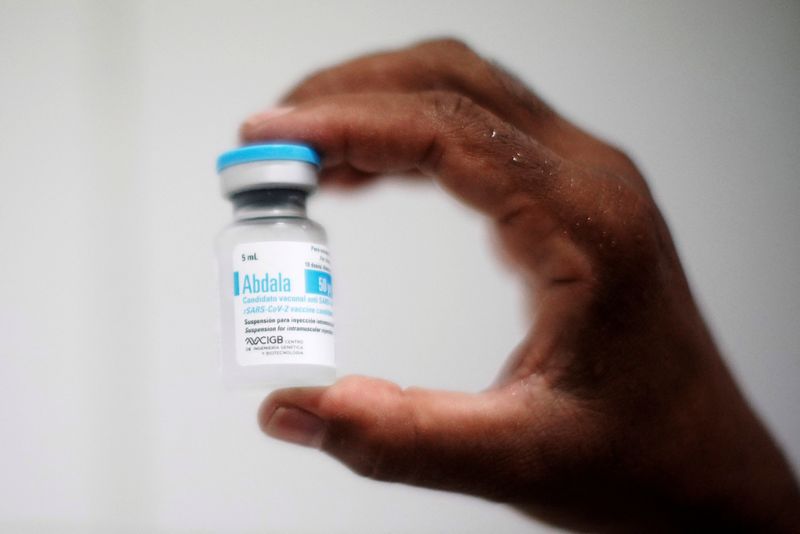
MEXICO CITY (Reuters) – Mexico’s health regulator Cofepris said on Wednesday it had authorized the Cuban-made COVID-19 vaccine Abdala for emergency use, even though the shot has still not been approved by the World Health Organization (WHO).
The vaccine received a “favorable technical opinion” from experts, Cofepris said in a statement.
Mexico has so far granted emergency use authorization to ten vaccines in total, including China’s CanSino and Sinopharm vaccines and that of U.S. pharmaceutical company Pfizer.
Cuban scientists have developed three homegrown vaccines against COVID-19, all of which are waiting to receive official recognition following an evaluation by the WHO, according to the island’s authorities.
Cuba has said its homegrown, protein-based vaccine Abdala is among the world’s most effective, with more than 90% efficacy.
Cuba, however, has not yet published results of its large-scale clinical trials in peer-reviewed journals.
Abdala has been authorized for use in some other countries, including Vietnam and Nicaragua.
(Reporting by Cassandra Garrison; Additional reporting by Diego Ore; Editing by Dave Graham)