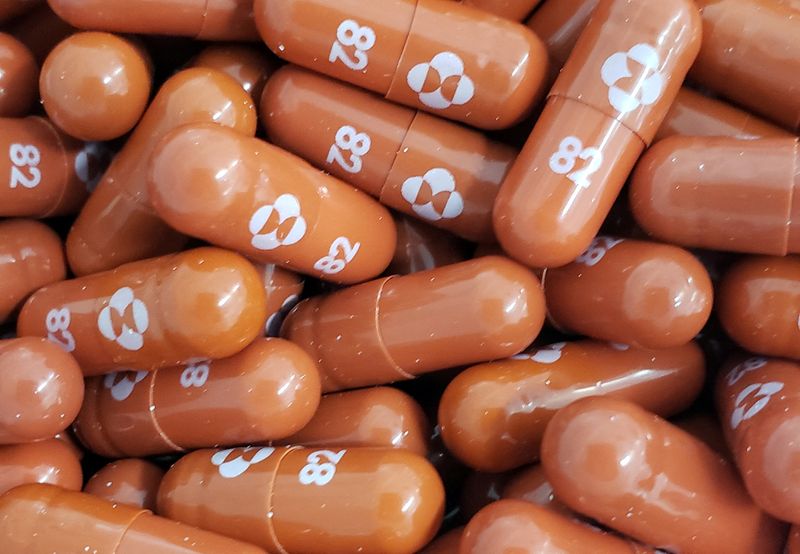
MEXICO CITY (Reuters) – Mexico’s health regulator has granted authorization for emergency use of drugmaker Merck’s COVID-19 pill Molnupiravir, President Andres Manuel Lopez Obrador said on Friday.
The health regulator, COFEPRIS, confirmed the approval in a statement later in the day.
COFEPRIS was expected to soon also approve Pfizer’s Paxlovid pill to treat COVID-19, Lopez Obrador added at a regular news conference. Both medications were approved last month in the United States.
Molnupiravir was developed with Ridgeback Biotherapeutics and shown to reduce hospitalizations and deaths by around 30% in a clinical trial of high-risk individuals early in the course of the illness.
Lopez Obrador said he planned to make both medications available in public hospitals.
(Reporting by Daina Beth Solomon; Editing by Dave Graham and Sandra Maler)