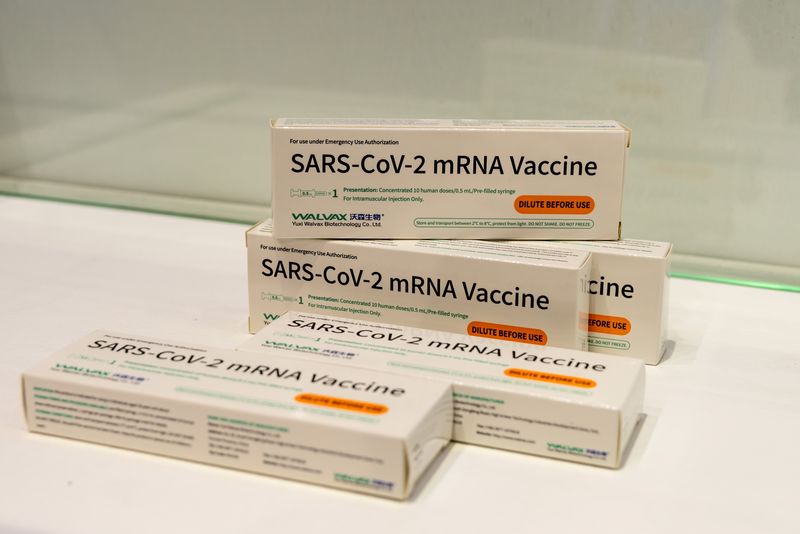
(Reuters) – Nepal has allowed China’s Walvax Biotechnology to conduct phase 3 trials for the company’s mRNA COVID-19 vaccine candidate in the country, China’s official Xinhua news agency reported on Friday, citing a Nepal health official.
The ARCoV vaccine was jointly developed by China’s Suzhou Abogen Biosciences, the Institute of Military Medicine under the Academy of Military Sciences and Walvax.
“We received a proposal from the Chinese company about a month ago seeking approval to conduct clinical trials,” Pradip Gyanwali, executive chief of the Nepal Health Research Council, told Xinhua. (https://bit.ly/3gGk8FH)
“Access to vaccines will be easier if they are manufactured in Nepal.”
Walvax and local partner Deurali-Janta Pharmaceutical Pvt Ltd will conduct the trials in Nepal on 3,000 people at BP Koirala Institute of Health Sciences, a government facility in the eastern city of Dharan, the report said.
(Reporting by Juby Babu in Bengaluru; Editing by Devika Syamnath)