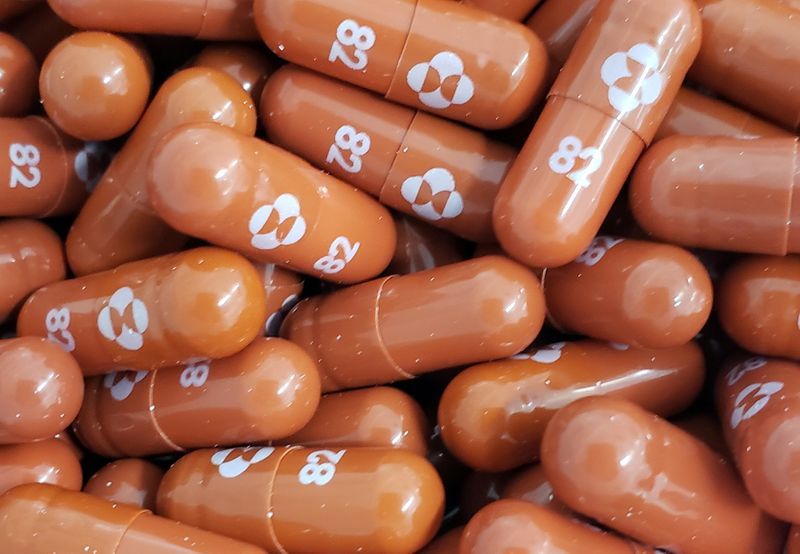
(Reuters) – A panel of outside advisers to the U.S. Food and Drug Administration on Tuesday voted in favor of authorizing Merck & Co oral COVID-19 pill, saying the drug’s benefits outweigh its potential risks.
(Reporting by Manojna Maddipatla in Bengaluru; Editing by Lisa Shumaker)